If you have no idea what inhibitors are, not to worry! This guide has got all you need.
What is an inhibitor?
An inhibitor is simply a molecule that binds to an enzyme to limit its activity. In other words, an inhibitor disturbs the activity of an enzyme. When these molecules bind to the active site of an enzyme, they decrease the level at which the enzyme and substrates are compatible with each other.
Thus, it is safe to say that when there is an increase in the concentration of an inhibitor, the level at which the enzyme carries out its activities reduces. This also means that as the concentration of these inhibitors increase, the amount produced decreases and vice versa.
This is why so many drugs in our world today are enzyme inhibitors because they can be effectively used to repair metabolic and hormonal imbalances or fight pathogens.
How does it work?
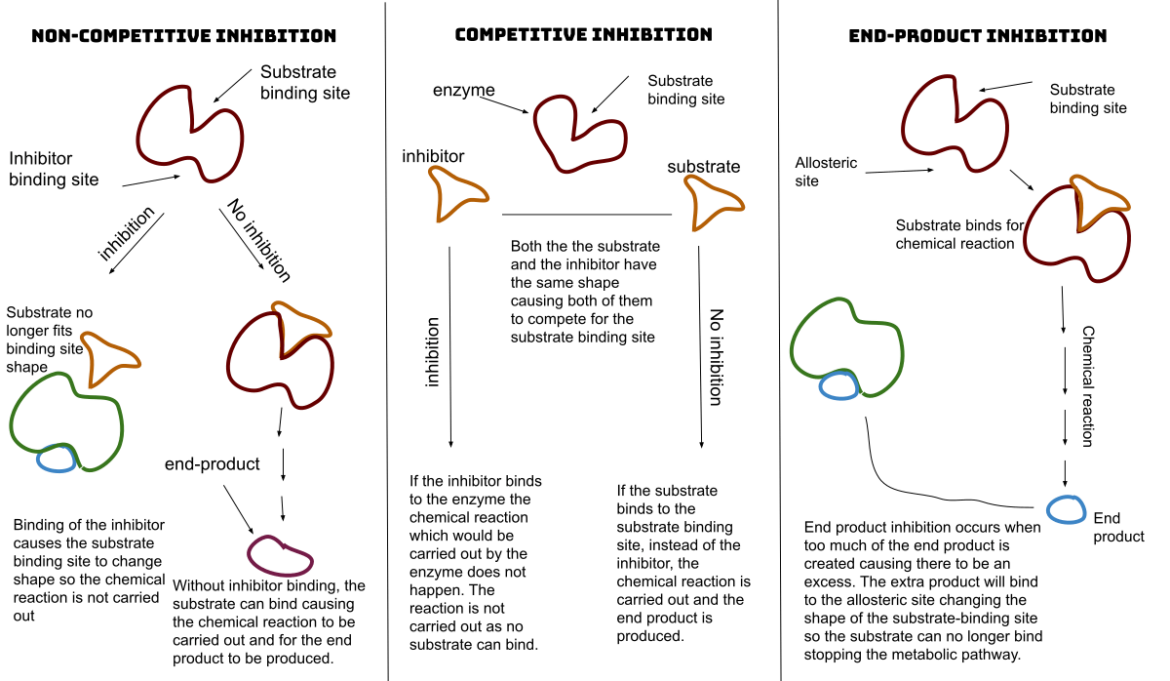
When an inhibitor attaches to an enzyme, it restricts substrates from penetrating its active sites or stops it from catalysis. Note that this attachment can be either reversible or irreversible. The difference is that irreversible inhibitors binds to enzyme and changes them chemically. This is often done through covalent bond formation.
On the other hand, reversible inhibitors attach non-covalently. You also have to note that inhibitions are produced based on whether they bind to the enzyme substrate, the enzyme itself, or both.
Since enzyme inhibitors are good drug molecules, they have been very useful in medical research, especially in the area of pharmacology and biochemistry. However, most people feel that enzyme-inhibitors are not specific and potent enough.
In other words, they claim that enzyme inhibitors do not bind to other proteins, and has an unstable dissociation constant (this ‘constant’ notes the concentration required for enzyme inhibition). Once it has high potency and specificity, researchers are sure that it will have a low toxic level and fewer side effects.
However, you have to note that not all drugs are enzyme inhibitors. For instance, anti-epileptic drugs restrict enzyme activity, which causes less of it to be produced.
Here are some important uses of inhibitors in today’s world:
- Antibiotics: Drugs use enzyme inhibitors to fight or reduce the spread of pathogens. If you have bacteria, you would normally be treated with vancomycin or penicillin.
What you don’t know is that the bacteria are surrounded by a thick wall of polymer known as peptidoglycan.
Penicillin and vancomycin now obstruct the enzymes and interlink the strands of the polymer with each other.
Antibiotics are created when an enzyme that supports a pathogen’s life is different or absent.
- Pesticides: A lot of pesticides are enzyme antibiotics. For instance, the enzyme acetylcholinesterase (AChE) can be found in animals. It aids appropriate nerve cell function when it breaks down the neurotransmitter, acetylcholine, into different parts- acetate and choline.
These AChE inhibitors are used in both agriculture and medicine.
- Chemotherapy: Since one of the greatest uses of inhibitors is to treat illnesses, they are widely used in common medical procedures, such as chemotherapy.